GI SYMPTOMS AND ASPIRIN DISCONTINUATION
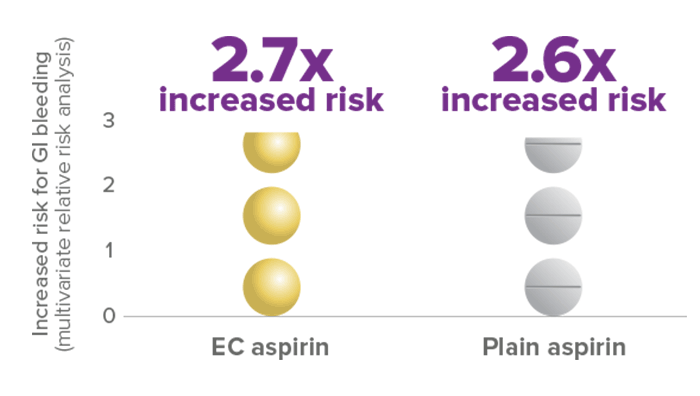
Enteric-coated (EC) aspirin shows a similar risk of upper GI bleeding vs plain aspirin
EC and plain aspirin (≤325 mg/day) have a comparable risk for GI bleeding6