MECHANISM
OF ACTION
Coordinated omeprazole and
aspirin delivery with Yosprala
Yosprala proprietary Intelli-COAT™ system is designed to sequentially deliver immediate-release omeprazole and delayed-release aspirin1,2
pH-sensitive coating dissolves at pH >5.51
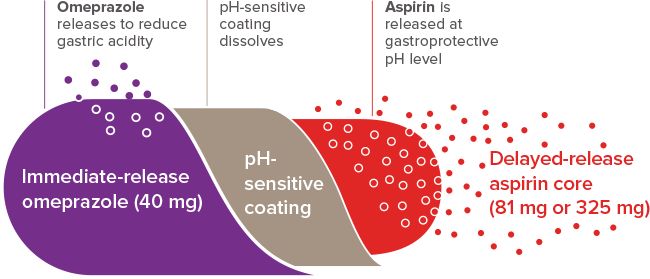
For illustration purposes only. Not a visual representation of the tablet.
• Yosprala is not interchangeable with the individual components of aspirin and omeprazole1